Flash Calculation
The isothermal flash calculation is one of the most important and most common calculations in any PVT calculation. The term flash is nothing more than taking some mixture (\(z_i\)) and equilibrating it at a specified pressure (\(p\)) and temperature (\(T\)). The resulting single or multi-phase mixtures (vapor \(y_i\) and liquid \(x_i\)), K-values (\(K_i=y_i/x_i\)) and the resulting volume(s) (or Z-factors) are the main outputs of the flash calculation.
The flash calculation is divided into three main parts: (1) the material balance, (2) calculating the component fugacities and (3) updating the K-values to try and reach thermodynamic equilibrium. The first part requires that the molar balance of each component is concerved. This is solved using the Rachford-Rice or Muskat-MacDowell equation. Part (2) requires the fugacities for each component in each phase to be calculated. This calculation is EOS dependent. The goal is to find a set of K-values that result in the equal fugacity constraint: \(f_{Vi}=f_{Li}\). The last part of the flash calculation considers how to update the estimate of K-values based on the current set of K-values and the fugacities.
The material balance equation, for which the Rachford-Rice and Muskat-MacDowell equations are based, can be written as \(z_i=y_i \cdot F_V + x_i \cdot (1-F_V)\), where \(z_i\) is the total composition, \(y_i\) is the vapor composition, \(x_i\) is the liquid composition, and \(F_V\) is the vapor molar fraction (\(F_V = \frac{n_G}{n_G+n_L}\)). The solution space for the flash calculation is divided into three regions: positive flash where \(F_V\) is between 0 and 1, negative flash where \(F_V\) is between 0 and \(-\infty\) or 1 and \(\infty\), and the third region is the trivial region where all K-values are equal to 1 and the value of \(F_V\) is arbitrary.
1. Generating a Flash Calculation
To perform a Flash Calculation, enter the temperature and pressure at which the reaction will occur, along with the composition of each relevant component. The complete process is shown in the GIF below.
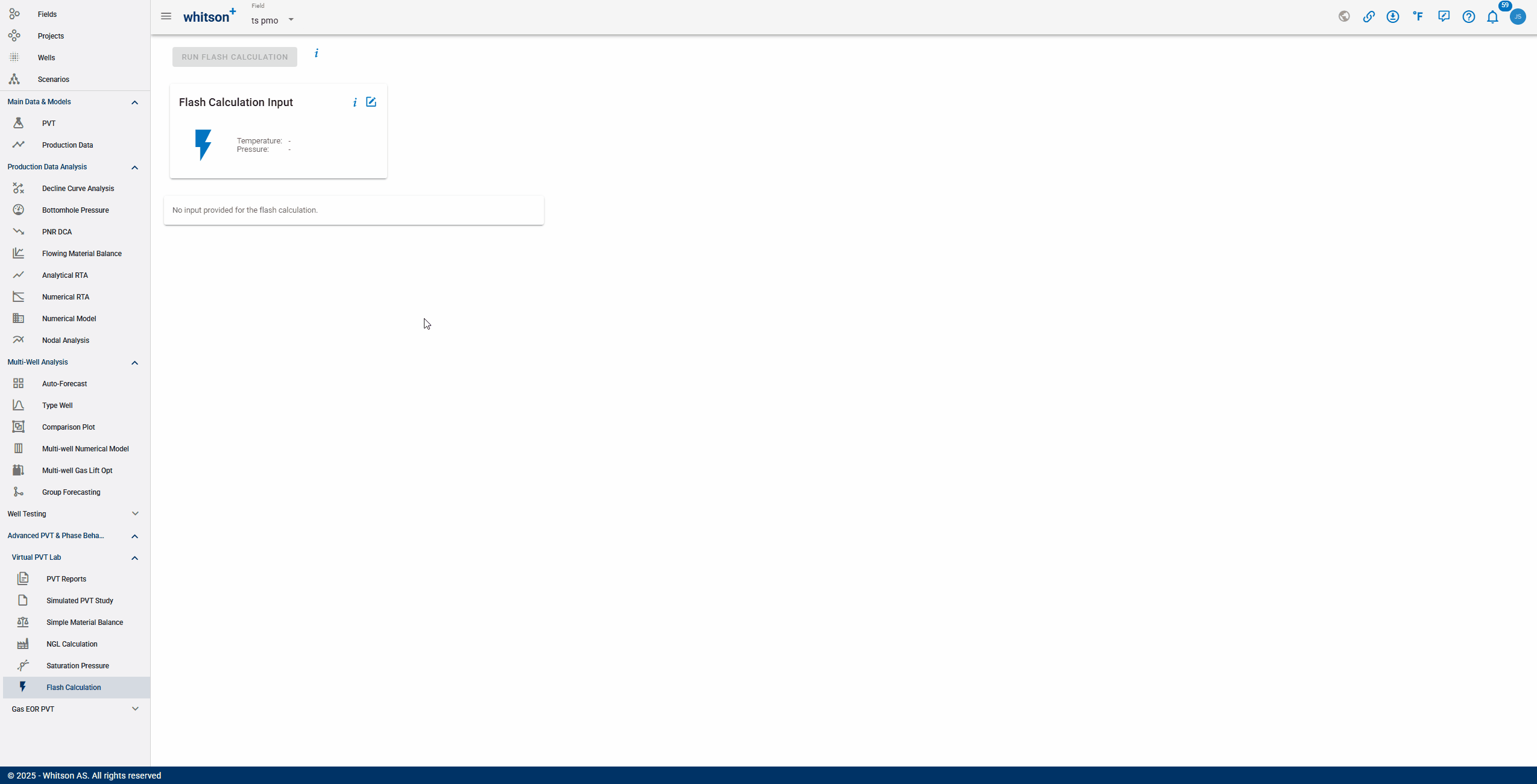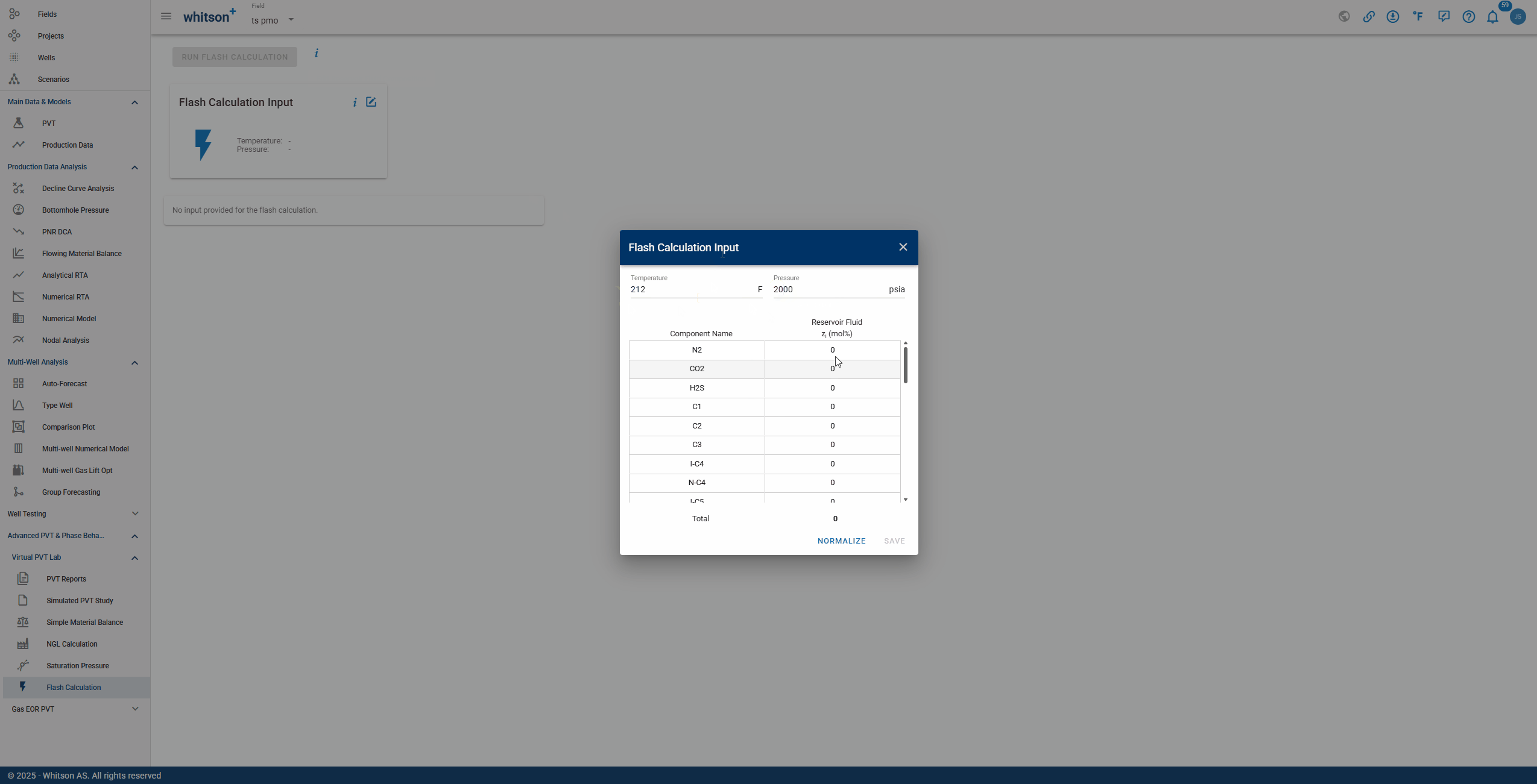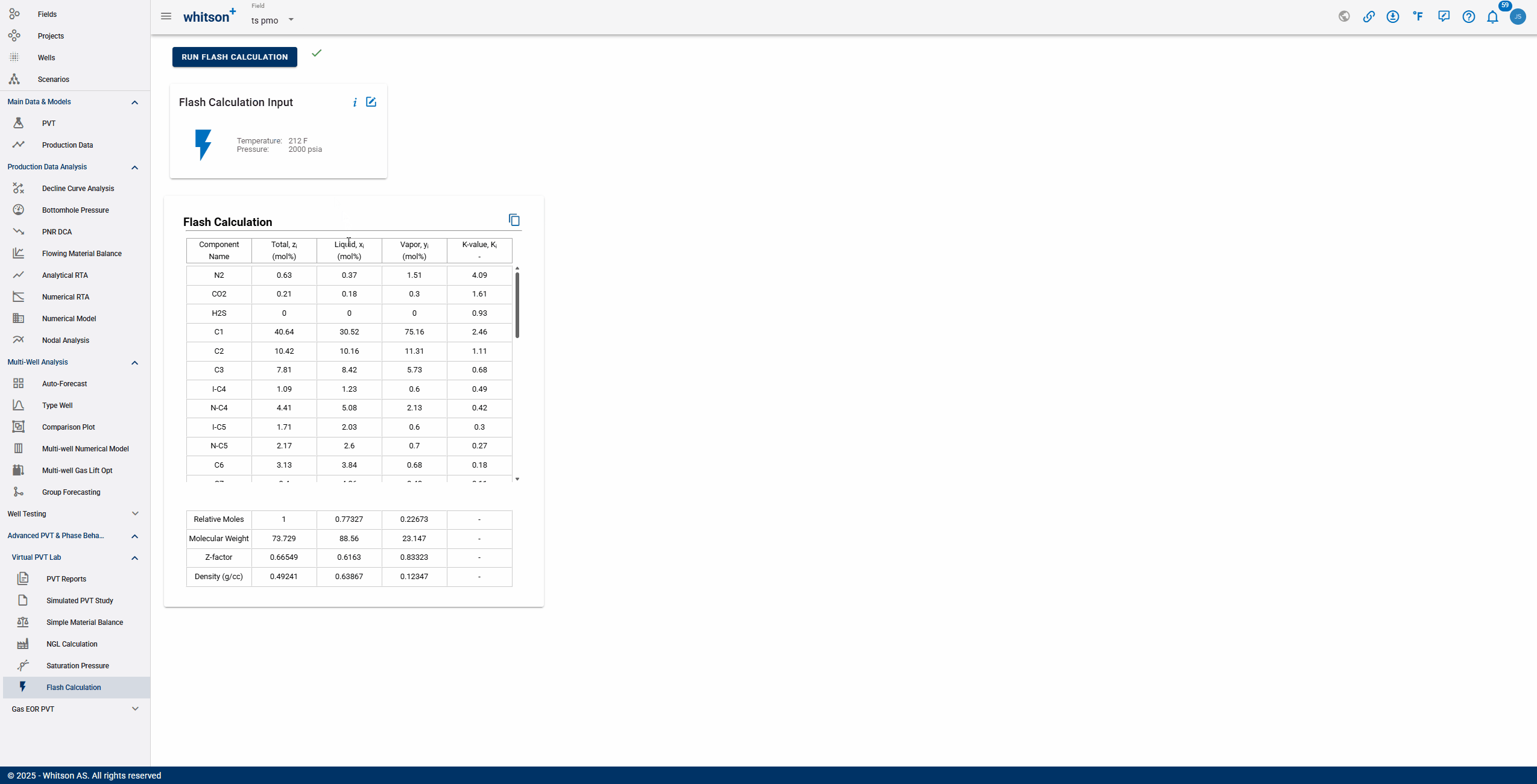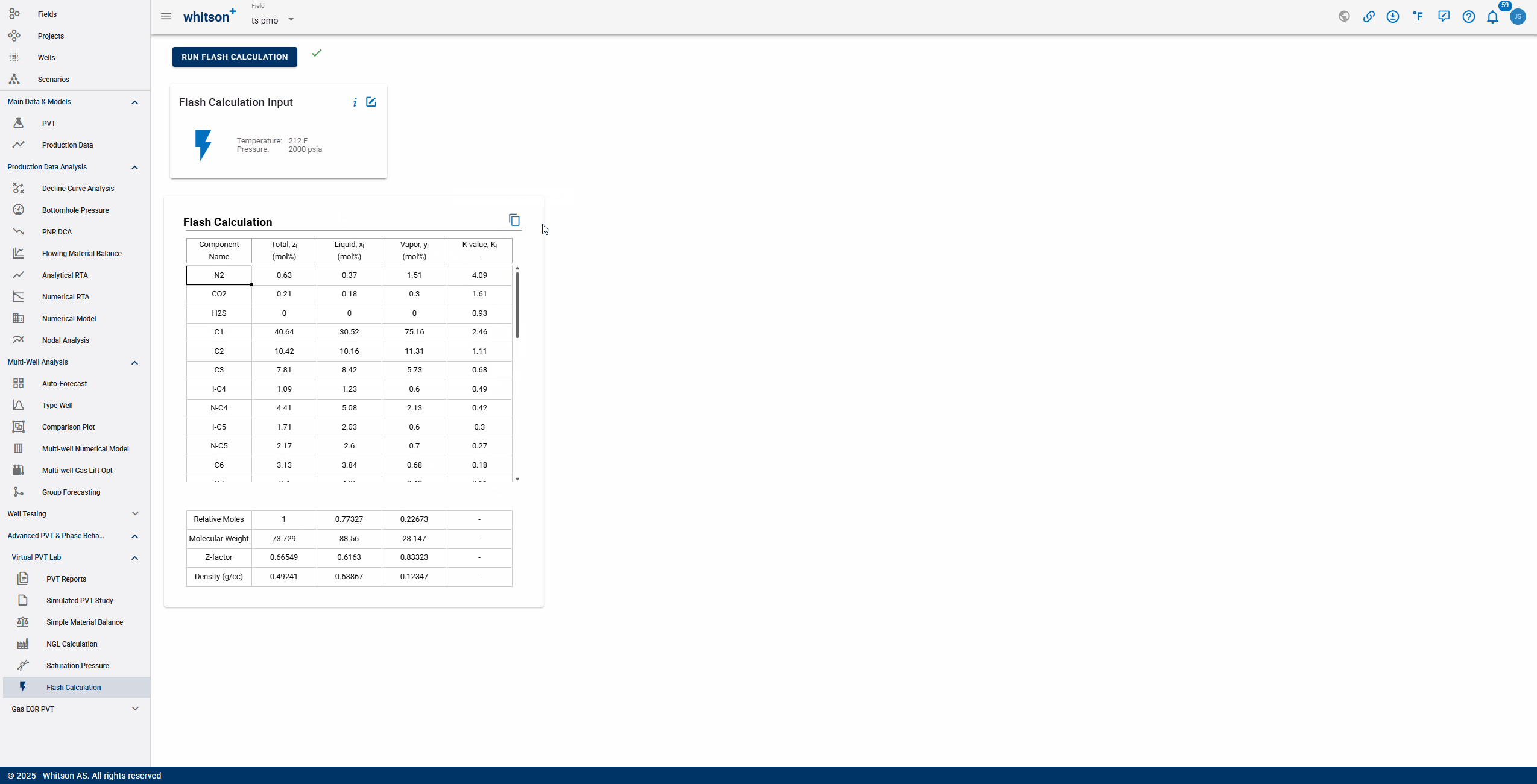
The Flash Calculation feature provides a comprehensive breakdown of a multicomponent mixture at specified temperature and pressure conditions. The output includes:
-
Component Composition
- Total mole fraction (): The overall fraction of each component in the feed.
- Liquid mole fraction (): The fraction of each component present in the liquid phase.
- Vapor mole fraction (): The fraction of each component present in the vapor phase.
- Equilibrium K-value (): The phase equilibrium ratio of each component, indicating its relative preference for the vapor phase compared to the liquid phase.
-
Overall Phase Properties
- Relative moles: The distribution of moles among phases, showing how the feed splits between vapor and liquid.
- Molecular weight: Average molecular weight of the total mixture and individual phases.
- Z-factor: Indicates deviation from ideal gas behavior for the mixture and each phase.
- Density: The calculated density for the liquid, vapor, and overall mixture, useful for further process calculations.